Research Focus
The focus of research has been for many years the investigation of the therapeutic role of Thyroid Hormone in tissue repair.
A part of the results of this research has been translated into clinical practice with the Phase IIa clinical study (Thy-Repair, EudraCT: 2016-000631-40). The Thy-Repair study investigates the effectiveness of triiodothyronine as a new therapeutic approach for tissue repair in patients with acute myocardial infarction undergoing reperfusion with primary angioplasty.
Since 2010, the focus of the team's research effort has been to investigate the potential of the thyroid receptor TRα1 as a new pharmacological target for tissue repair/regeneration. These studies have shown for the first time that: a) myocardial stress leads to the translocation of TRα1 to the nucleus and converts it into a molecular regulator of tissue repair according to existing T3 levels, b) T3 exerts a cardioprotective effect on ischemia-reperfusion injury through activation of the cytosolic TRα1 receptor, c) Downregulation of the nuclear TRα1 receptor as well as the administration of a specific inhibitor of TRα1 after acute myocardial infarction result in a significant worsening of heart failure.
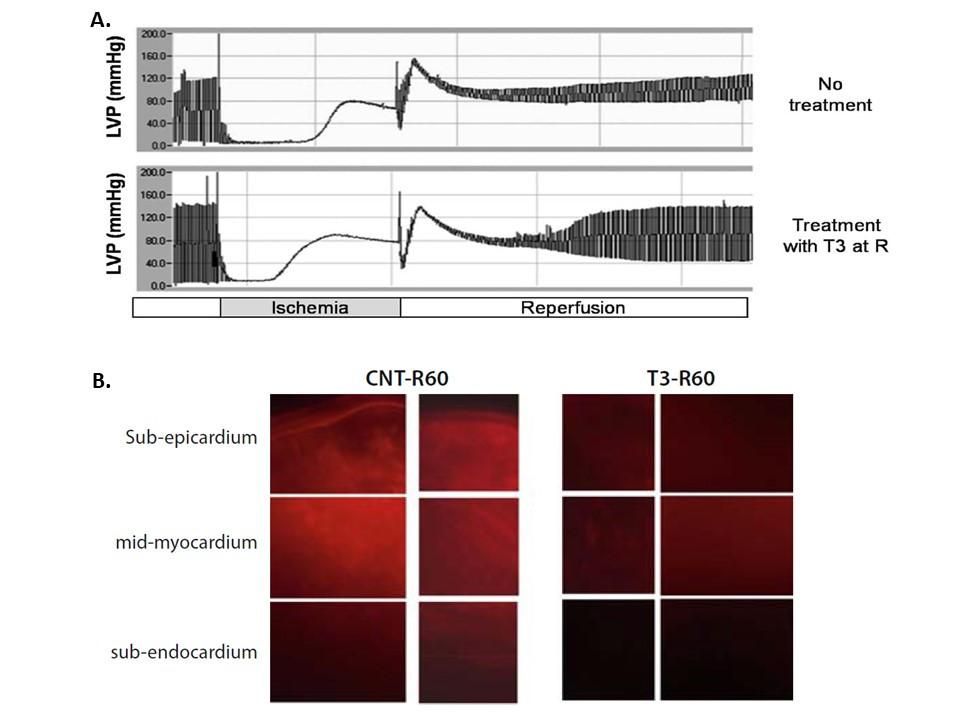
Figure 1. TH improves cardiac hemodynamics while limits apoptosis in the setting of ischemia–reperfusion. This may be a unique paradigm of an agent with anti-apoptotic actions. (a) Langendorff recordings of left ventricular pressure from isolated rat hearts subjected to ischemia-reperfusion without treatment (non-treated) and with T3 treatment (40 lg/l) at reperfusion (T3-treated). (b) Microscopy images showing caspase-3 activity detected by fluorescent probe in non-treated and T3-treated hearts. The probe was a generous gift from Quidd (France). (Heart Fail Rev (2010) 15:143–154)
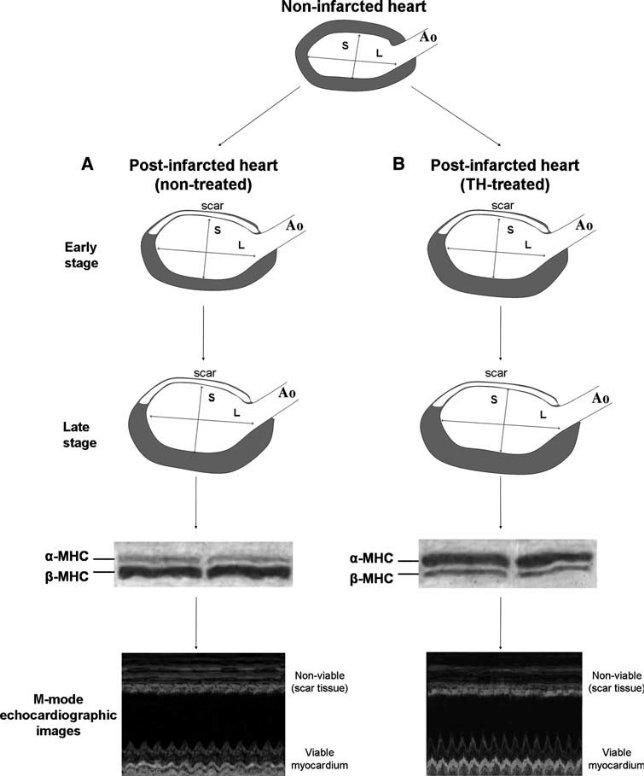
Figure 2. Schematic of changes in left ventricular shape and wall tension after myocardial infarction in TH and non-treated hearts. (a) Left ventricular chamber becomes dilated and spherical in shape in non-treated post-infarcted hearts, while hypertrophy develops at late stages. The ratio of maximal long axis (L) to maximal short axis (S) of the left ventricle is reduced and the expression of β-myosin heavy chain (MHC) increases while α-MHC decreases, which results in reduced contractile function of the viable myocardial segment. (b) TH treatment reshapes left ventricular chamber towards a more ellipsoidal shape. Hypertrophy develops earlier with increased α-MHC and decreased β-MHC expression and results in normalization of the wall stress at early stages. Contractile function of the
viable myocardium assessed by echocardiography is markedly improved. Ao = Aorta (Heart Fail Rev (2010) 15:143–154)
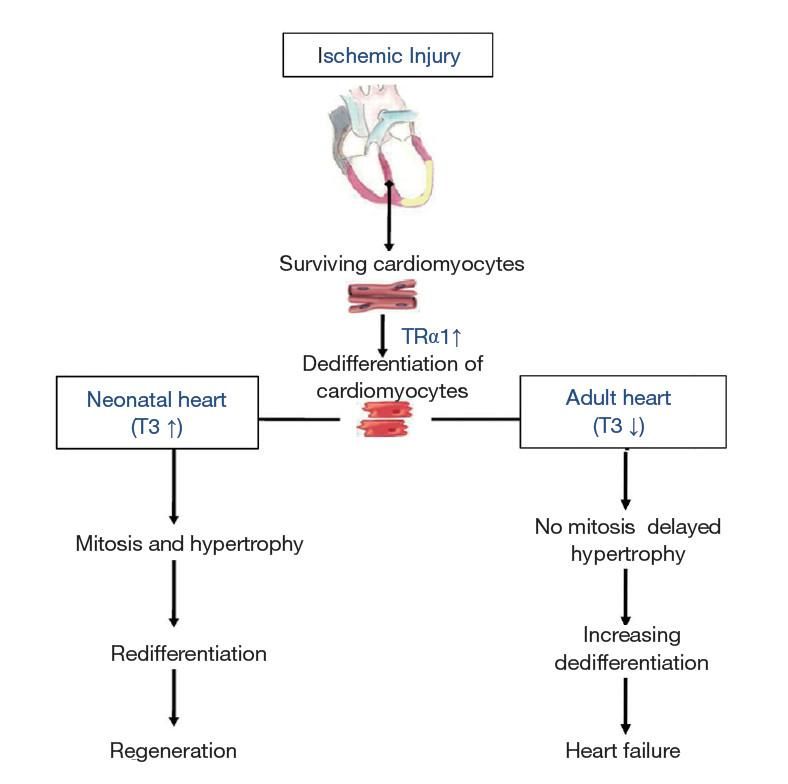
Figure 3. Changes in thyroid hormone signaling determine the response of the heart after ischemic injury. The initial response of the adult heart to injury involves enhanced expression of thyroid hormone receptor α1 (TRα1) contributing to dedifferentiation. In this context, low T3 blocks the regenerative response and leads to increasing dedifferentiation, dysfunction and heart failure. However, high T3 levels combined with increased expression of TRα1 (neonatal heart) lead to increased cardiac mass (proliferation and hypertrophy), redifferentiation and regeneration. ↑, increase; ↓, decrease (Ann Transl Med. 2018 Jun; 6(12): 254.)
These results led to the design and synthesis of triiodothyronine analogues with potential selective agonist activity for TRα1 receptor.
At this stage the main research activity of the team focuses on the following innovative fields:
1. The phase IIa clinical study (Thy-Repair, EudraCT: 2016-000631-40) was completed in December 2020 with positive results in cardiac function and cardiac remodeling. Our research team is designing and conducting a multicenter phase IIb /III clinical trial in collaboration with Uni-Pharma Pharmaceutical Laboratories to demonstrate the beneficial effects of T3 on patients with myocardial infarction undergoing revascularization.
2. Study of T3 regenerative effects immediately after myocardial infarction in rats in vivo. This study aims to investigate new actions of T3 and the in-depth understanding of the mechanisms related to the results of the clinical study Thy-Repair. The protocol is analogous to the clinical study and investigates the effect of different doses of T3 on the functional, healing and regenerative response of the heart after ischemia. Immunohistochemistry and immunofluorescence techniques are used.
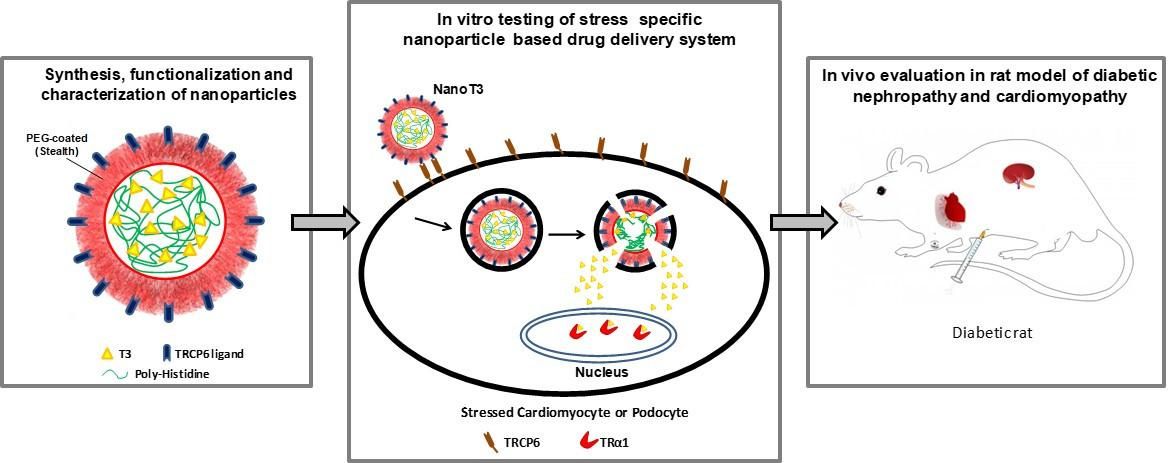
3. Development of T3 nanocarriers that selectively target stress-induced myocardial cells. These nanocarriers will be able to transfer the necessary therapeutic amount of T3 to stressed cells (that overexpress TRα1) while not affecting healthy cells reducing potential side effects. This research is funded under the European Research Program a. "Regenerating the diabetic heart and kidney by using stress-specific thyroid hormone nanocarriers" (EURONANOMED2019-049 / REASON)
Figure 4. Concept of design and action of T3 nanocarriers in diabetic cardiomyopathy and nephropathy.
4. Design and synthesis of triiodothyronine analogues with possible selective agonist activity for TRα1 receptor. The goal of selective TRα1 receptor agonists is to induce stronger cardioprotection against ischemic injury and tissue repair / regeneration with fewer side effects from other systems. These new agents are tested in culture models of myoblasts and cardiomyocytes and in experimental models of myocardial damage and heart failure in the context of 2 doctoral dissertations.
5. Pharmacological methods for protection of heart transplants. Cold heart cardioplegia is currently the method of choice for protecting and transporting implants during heart transplantation. This method, however, ensures short-term cardioprotection. Alternatively, the implants can be transferred to a special warm perfusion device (organ care system, Transmedics Endover, MA, USA) with simultaneous monitoring of the functional parameters. However, this method requires an perfusion solution that contains high cost ingredients and needs to be mixed with oxygenated donor blood which complicates the method. Our research group has developed a special solution for long-term ex vivo preservation of heart transplants with simple composition and low cost that does not require mixing with oxygenated blood.
6. Study of the actions of T3 as a therapeutic approach for microvascular dysfunction and multiorgan failure caused by sepsis. Studies in the myocardium have shown a significant effect of T3 on microvascular damage after myocardial infarction. This action can also be beneficial for microvascular dysfunction caused by sepsis. The study is performed in an in vivo model of ligation and perforation of the cecum in mice with the induction of peritonitis and is done in collaboration with the company Uni-Pharma Pharmaceutical Laboratories. The presence of tissue hypoxia in various organs is studied with an innovative method of immunohistochemistry based on pimonidazole.
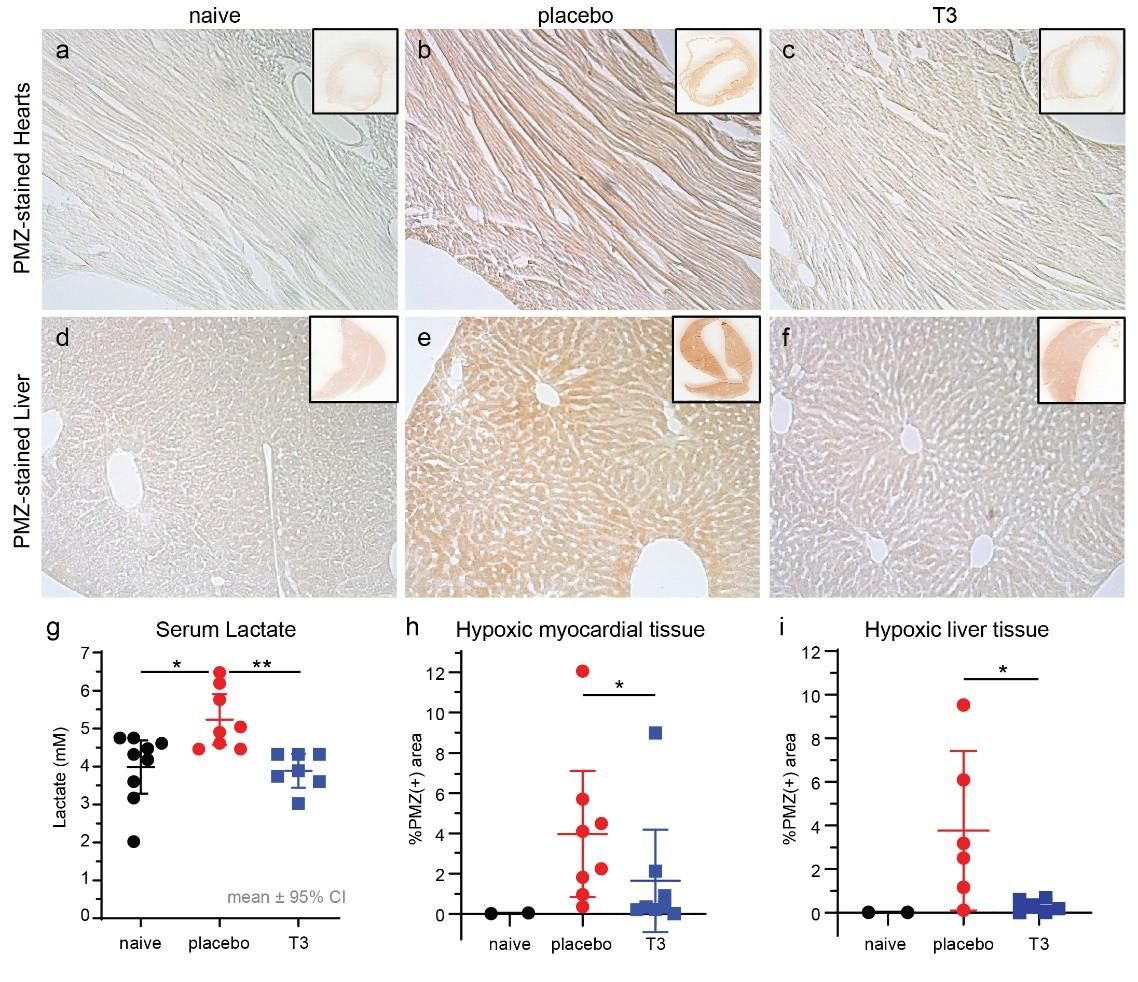
Figure 5. Representative microscopy images after Pimonidazole (PMZ) staining from heart and liver tissues of naive (a), placebo (b) and T3-treated (c) animals. Detection of hypoxia (PMZ positive) was based on immunoperoxidase staining (brown colour). (d) Lactate levels in serum were 5.2±0.28 mM in placebo and 4.2±0.35 in T3 treated group 18 hours after surgery, p<0.05. Baseline lactate levels in naive animals were 4.1±0.3 mM, p<0.05 vs placebo only. (e) Quantification of PMZ-positive staining in left ventricular tissue was 4%±0.5 in placebo and 1.5%±0.5 in T3 treated hearts 18 hours after surgery, p=0.028. (f) PMZ-positive staining in liver was 3.8% ±1.4 in placebo and 0.3%±0.1 in T3 treated hearts, p=0.026. (Intensive Care Med Exp. 2021 Dec; 9: 17)
Results are presented as mean±SEM, . * p<0.05 vs placebo
Contact Person #1
Contact Person #2